TiNT Trial: Trametinib in NF Tumours
May Update
overview
Neurofibromatosis type 1 (NF1) is a common genetic condition, affecting 1 in 2,500 Australians, which can cause a number of medical issues ranging in severity. Some children and young adults with NF1 develop tumours, called low grade optic pathway gliomas (OPG) or plexiform neurofibromas (PN).
Although survival is high, many patients with OPG or PN suffer from visual loss, pain, disfigurement and brain impairments. These life-long and highly debilitating effects suffered by NF1 patients motivated Australian and New Zealand researchers to develop the TiNT study. The trial will evaluate the use of trametinib, a MEK inhibitor that reduces tumour growth in adults diagnosed with melanoma, and has shown promising results in preliminary studies with neurofibromatosis tumours.
The TiNT trial is the first trial in the world to comprehensively examine if treatment with trametinib can reduce tumour growth in children and adolescents experiencing NF-associated tumours. Other Quality of Life (QoL) aspects will also be measured, including pain, vision and memory. Additional data will be gathered on safety, event-free and overall survival.
The TiNT trial will be available at every children’s cancer centre throughout Australia and New Zealand. ANZCHOG is the national sponsor for TiNT at all participating sites, with ANZCHOG’s National Trials Centre providing central coordination and oversight for the trial. The Murdoch Children’s Research Institute (MCRI) will lead the neuropsychology and statistical elements of the trial.
Associate Professor Geoffrey McCowage and Dr Andrew Dodgshun jointly lead the TiNT trial. A/Prof McCowage is an experienced oncologist at the Children’s Hospital at Westmead and Dr Dodgshun is a paediatric cancer specialist at Christchurch Hospital, New Zealand. Associate Professor Johnathan Payne is the lead neuropsychologist for the neurocognitive and quality of life (QoL) components of the trial.
TRIAL UPDATE
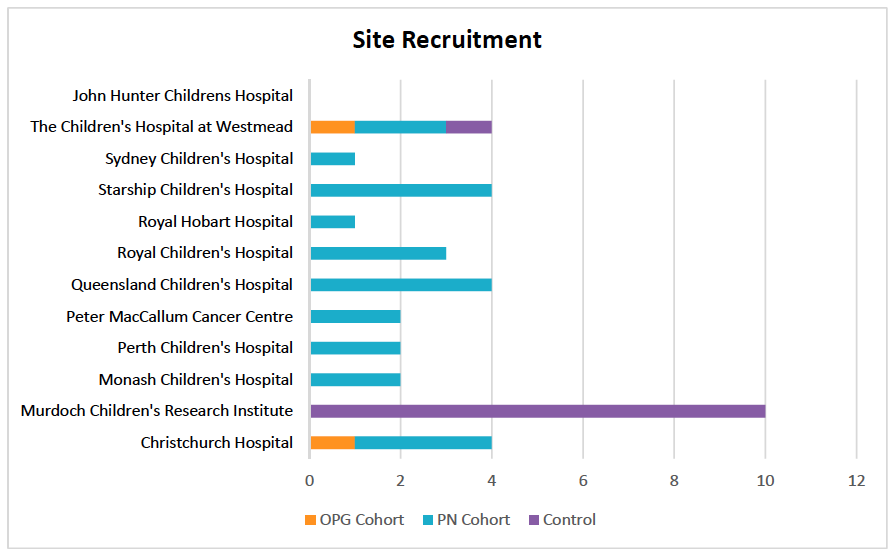
TiNT is recruiting at twelve centres across Australia and New Zealand with the final site to open in the coming months. The team are aiming to recruit 120 participants in total, with 60 patients to receive trametinib in two treatment arms equally distributed between PN and OPG participants, and 60 control participants. Thus far:
- 24 participants with PN have been enrolled and are receiving trametinib
- 2 participants with OPG have been enrolled and are receiving trametinib
- 11 participants have been enrolled on the control arm.
To read the full report, click here:
May 2022 TINT TRIAL UPDATE