It is estimated that around half of all people who develop NF1 or NF2 inherit it from a parent. The remaining 50% will develop it by chance, as the result of a spontaneous change in a specific gene in an egg or sperm cell.
Every person affected by NF1 or NF2 has a 50% chance of passing the condition on to their offspring. Schwannomatosis is less well understood, but the majority of cases appear to occur by chance, not because they are inherited.
WHAT ARE GENES?
We can think of genes as being the chemical instructions that tell our cells (our body’s building blocks) what to do. They are made up of long strands of DNA and are responsible for making all the proteins our bodies need to function normally.
We all have around 22,000 genes in our bodies. These genes are housed in ‘books’ called chromosomes. There are 46 chromosomes, which is 23 pairs of chromosomes: 22 pairs of autosomes and 1 pair of sex chromosomes. We inherit one copy of each chromosome and therefore gene from each of our parents.
For more information about genes check out the Garvan Institute’s video: DNA, Genes & Genomes
GENETICS OF NF
Each form of NF is caused by a different gene found on two different chromosomes. A single gene is responsible for causing the variable signs and symptoms we see in NF1 and NF2. These genes are located on chromosome 17 for NF1 and 22 for NF2 respectively.
Schwannomatosis is different in that the genetic basis for it is still uncertain, although studies in large families impacted by this condition have revealed two genes, LZTR1 and SMARCB1 as being involved in causing the condition. These two genes are located on chromosome 22 as well, not far from the NF2 gene.
HOW IS NF INHERITED?
NF can arise in any family and around half of the people affected will have a parent with the condition. The other 50% arise as new cases caused by a spontaneous change (mutation) to one of the NF genes.
The image below is a representation of how NF is inherited.
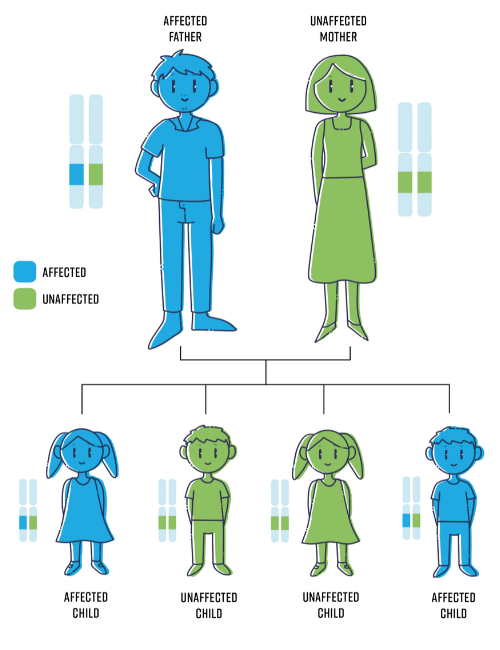 NF does not skip generations which means you cannot be a carrier if you do not have it, even if one or more parents have NF. NF1 and NF2 are therefore said to have “complete penetrance”.
NF does not skip generations which means you cannot be a carrier if you do not have it, even if one or more parents have NF. NF1 and NF2 are therefore said to have “complete penetrance”.
Schwannomatosis is different to NF1 and NF2 in that not everyone with the gene change will develop tumours or other symptoms of the condition. This is referred to as incomplete or reduced penetrance.
NF1 and NF2 are autosomal dominant conditions
As NF is an autosomal dominant condition, a person only needs one copy of the changed gene to have the condition. So, when we receive the copy of our NF gene from our affected mum or dad, they will pass on either:
- An unchanged copy of the NF gene -> unaffected
- A changed copy of the NF gene -> affected
Therefore, there is a 50% chance of inheriting the condition from a parent. You might think of it as like tossing a coin for every pregnancy/child a couple has.
This means that, regardless of whether a person inherited NF from their parent or whether it was a new mutation in them, there is then a 50% or 1 in 2 chance that they will pass the condition on to a child.
Schwannomatosis is more complex and seems to involve more than one gene.
While most cases appear as new gene changes (mutations), families with extensive history have shown a dominant inheritance pattern like NF1 and NF2. It is therefore thought that once someone has Schwannomatosis the chance of their child having the condition is 50% or 1 in 2.
MOSAIC OR SEGMENTAL NF
Segmental NF (sometimes referred to as mosaic) is a form of NF1 or NF2 in which signs and symptoms are limited to only one area (segment) of the body.
For people with this diagnosis the chances of passing the condition onto their children is thought to be as low as it is for the general population, but this depends on which area of the body is affected. If the area includes the ovaries or testes, the chances of passing it on could be as high as 50%.
Our US counterparts have an excellent information sheet Segmental NF: A guide for patients.
GENETIC TESTING
Genetic testing is available for the NF genes and can be useful to:
- Confirm a diagnosis
- Assist with family planning
There is currently only a handful of known links between the change to the NF gene someone has and the way that the change affects them. In general, genetic testing is not used to inform monitoring or management and is not routinely offered to patients for this purpose.
Can symptoms be predicted?
At this stage there are only emerging trends between genotype and phenotype correlations with NF1. This means that it is hard to predict how a person will be impacted by NF based on what the geneticists see in their gene sequences.
The severity of NF also cannot be predicted by the NF symptoms in a parent. Therefore, genetic testing is not used at this point to make management or clinical decisions. These are based on International Clinical Guidelines.
Speak to your local genetics service about whether testing will be funded for you or to find the costs involved to self-fund. The costs will vary.
Testing to Confirm a Diagnosis
Genetic testing may be employed to confirm a diagnosis of NF. When a diagnosis of NF is suspected, this is often because a child or adult fulfills one of the required two diagnostic criteria. In the case of NF1 for example, a genetic test may be ordered to try to confirm the diagnosis. A blood or saliva sample can be used to extract DNA for analysis. This can be done in a couple of ways and can look at the suspected NF gene in isolation, a selection panel of genes known to cause similar signs and symptoms, or the whole genome (all your DNA) at a high or detailed level depending on the presenting signs and symptoms.
This testing is very good at picking up gene changes, but will not pick up every single change, and so while testing may confirm a diagnosis it will never completely exclude a diagnosis of NF1, but can indicate a diagnosis is less likely. The testing is considered to pick up 97% of gene faults. This means a change will be found in 97 out of 100 people with clinically confirmed NF who have a genetic test.
Family Planning
Genetic testing is also available to assist with family planning where a couple wishes not to pass on the faulty NF gene and therefore the condition.
Genetic testing to first confirm the diagnosis in the affected person must be undertaken before further tests are available for this purpose.
Testing is then available either during pregnancy or prior to pregnancy alongside IVF. More information can be found in the Adults – Living with NF section.
Impact on Insurance
Some people are concerned about whether having a genetic test will impact on their current and future insurance policies and requests.
Health Insurance does not take into account your current or risk of future health conditions when assessed. Therefore, genetic testing will not impact upon this insurance.
Life Insurance can be impacted by genetic testing results. While you cannot be asked to have genetic testing as part of a life insurance policy you are required to declare any past and current medical conditions. As NF is most often diagnosed clinically, (rather than via any genetic testing) having the condition will impact the risk assessment but should not prohibit you in most instances for obtaining/retaining a policy. For more information see the Centre for Genetics Education Factsheet on Genetic Testing and Insurance.
DOES THE TYPE OF GENE CHANGE AFFECT THE IMPACT OF NF?
For the majority of the gene faults seen in NF no relationship between the mutation and the way someone is affected has been found. This is called the genotype-phenotype correlation as explained above. However, there are a few exceptions to this.
It is also important to be aware that there is currently still very limited research literature in this area, and so much is not understood, this is why scientists describe these findings as “emerging trends”.
NF1 Whole Gene Deletions
Also referred to as 17q11 microdeletion syndrome, this can be a more severe form of NF1 impacting up to 5% of people with NF1. The characteristics of someone with their whole NF1 gene deleted may include, among others:
- Coarser facial features
- Developmental delay
- Intellectual disability
- An increased risk of cancers
- Larger than average number of neurofibromas
- Heart abnormalities
It is also important to remember that, like NF1, everyone with this deletion will have different signs and symptoms and will not necessarily develop all of those listed above or other known symptoms of NF1. The features of NF1 will also be experienced across a varying degree as they are for everyone with NF1.
For anyone with a copy of the NF1 gene deleted, it is important to remain under close surveillance for tumour burden and complications. However, it does not mean they will experience all the above symptoms.
Some people impacted by NF1 also show physical signs and symptoms like those of Noonan Syndrome, as well as some of the typical NF1 features. They are most likely to have:
- Wide-set eyes (hypertelorism)
- Enlarged and thickened heart muscle
- Low-set ears
- Multiple cafe-au-lait marks
- Drooping upper eyelid (ptosis)
- Narrowing of the valve connecting the heart to the pulmonary artery (which takes blood to the lungs)
- Short stature
- Webbed neck
- Specific learning disabilities
They may also have other signs and symptoms seen in either NF1 or Noonan Syndrome.
There are however some symptoms common in NF1 that are generally infrequently seen in people with this condition. These include:
Research has shown that a change within the NF1 gene is responsible for this presentation of the condition rather than that which causes Noonan Syndrome.
The doctor may send you for investigation by a cardiologist if these above clinical features are present.
Other Known Presentations of NF1
There are only a handful of known links between changes to the NF1 gene and its presentation.
Because the NF1 gene is a really long one, compared to many other genes in our bodies, it is more prone to faults (mutation). We know that there is a vast number of ways in which the gene can be changed and often the change identified when someone’s DNA is analysed has not been seen before.
This means there is often only minimal information, if any, about how a gene fault may impact someone, and so advice on this basis is generally provided with caution.
Your NF specialist or geneticist will be best placed to discuss your gene fault and whether it will affect your care.
Familial Spinal Neurofibromatosis
People affected by this condition generally have café-au-lait marks, limited neurofibromas on the skin and neurofibromas along the spinal cord.
NF1 Mutations
A few gene faults have now been linked to condition symptom severity aside from those indicated above. One such involves a small deletion of part of the gene which results in no dermal, sub-dermal or superficial plexiform neurofibromas developing, and therefore a milder form of the condition.
NF2 Mutations
A number of NF2 gene faults have been detected and some relationship between the type of fault and condition severity has been made, however, as there is still considerable variability in severity between people affected by similar faults the literature advises caution in providing families with the details of the impact of these changes.
Furthermore, the changes will not impact upon the way surveillance or management/treatment is undertaken other than to ensure that those people with a likelihood of more severe symptoms be followed closely over time.
Schwannomatosis Mutations
A correlation between pain and a change in the LZTR1 gene has been proposed for this condition. However, no links have been established between gene faults and tumour burden that we are currently aware of.
.png)